CONFIDENTIAL
Remote Decentralized
Clinical Trials Operating System
for Trial@Home(CLINTOS)
Acronym: CLINTOS
Call ID & Topic: ID: ECSEL-2020-3-IMI-ECSEL IMI-ECSEL Joint Activity Trials@Home

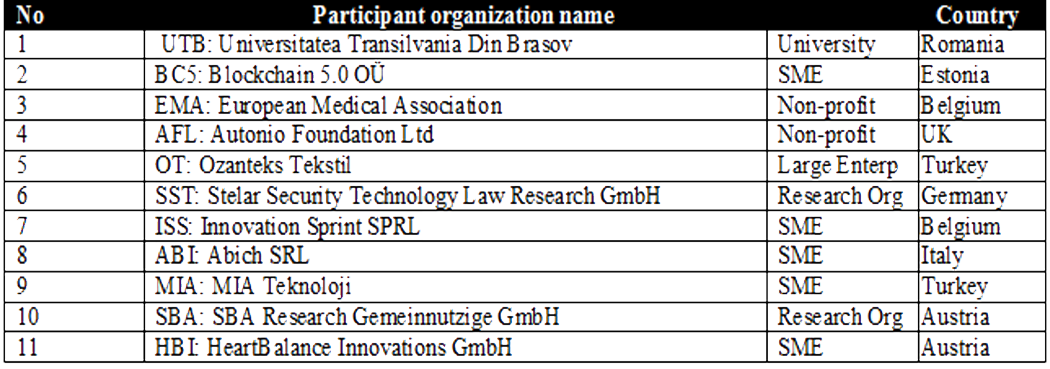
Special Trials@Home Advisory Board*
 *These partners are also participants of the original Trials@Home flagship project
funded under IMI Joint Undertaking (JU) and will ensure that this project will be “timed and
tuned to facilitate a close complementary activity between Trials@Home.
*These partners are also participants of the original Trials@Home flagship project
funded under IMI Joint Undertaking (JU) and will ensure that this project will be “timed and
tuned to facilitate a close complementary activity between Trials@Home.
Abstract
Clinical trials (CTs) are means of validating & receiving regulatory approvals for marketing new medical products for prevention, treatment & diagnosis of diseases. The average cost of bringing a new drug to market is estimated at $2.6 billion, with 2/3 of the cost going to CTs, about 90% of which fail. Moving CTs from the traditional clinic setting to the participant's immediate surroundings not only affords cost savings, but also makes it easier for larger, more diverse & remote populations to participate in CTs, enabling participants to visit a CT center less frequently, or not at all.Remote Decentralized Clinical Trial (RDCT) is a promising new way to conduct CTs that also maximizes stakeholder engagement as investors, physicians, state, patient advocacy groups, and even the patients themselves can play a role in study design, implementation, analysis & optimization of the remote monitoring approach.
Despite many benefits, RDCTs pose a number of operational & regulatory challenges including privacy, security & interoperability (PSI). Most CT systems lack capabilities in securing, anonymizing & making acquired patient data interoperable. Almost all health data still exists in silos at the risk of privacy/security breaches. The RDCT-as-a-Service presented in this proposal builds upon a pending PSIaaS (PSI-as-a-Service) & 2 granted H2020 (XENO & COVID) projects, using wearable sensors that broadcast vital signs in real time. This decentralized patient-centered approach integrates PSI by design into any existing CT system, wherein patients remain in full control of their personal online data (POD), authorizing, access to HMS, CDMS of EHR, via APIs when required. Thus, this novel Trials@Home framework functions as a universally compliant CT Operating System (CLINTOS) of future RDCTs, seamlessly connecting with the entire care continuum as plug-n-play federated cloud ecosystem generating new financial opportunities for partner SMEs in the multi-billion CT industry.

Our Vision:
To build a versatile, future-ready, user-centric, cloud based universally compliant Operating System for Trials@Home.
Our Mission:
To exploit ICT & wearable technologies for enabling a decentralized user-centric ecosystem for conducting RDCTs (Remote Decentralized Clinical Trials).
Our Goals: To build, test, validate CLINTOS (Clinical Trial OS) as RDCT-as-a-Service platform, and disseminate & exploit the cloud business model.
To build a versatile, future-ready, user-centric, cloud based universally compliant Operating System for Trials@Home.
Our Mission:
To exploit ICT & wearable technologies for enabling a decentralized user-centric ecosystem for conducting RDCTs (Remote Decentralized Clinical Trials).
Our Goals: To build, test, validate CLINTOS (Clinical Trial OS) as RDCT-as-a-Service platform, and disseminate & exploit the cloud business model.